Research Interests
- Nanostructures Tailoring:
Nanomaterials with unique morphology including nanoflowers, nanoporous, dendrites, and nanofibres are synthesized via different synthesis approaches such as electrochemical, chemical, wet-chemistry.

- Polymer Electrolyte Membrane Fuel Cells:
Fuel cells are an auspicious alternative renewable energy technology for the limited and environmentally harmful fossil fuels, thanks to their higher efficiency and lower emissions. Unfortunately, the high cost and poor performance of their commercial Pt-based electrodes obstruct their worldwide commercialization. Hence, My research group focuses on reducing the fuel cells cost without sacrificing their performance. This is achieved by the design of robust non-precious nanostructured electrocatalysts with unique morphologies (e.g., flowers, cubes, nanoplates, etc) as alternatives for such costly catalysts. Besides, we use advanced spectroscopy techniques to observe the catalytic processes in-situ to get a detailed chemical information about the catalyst–molecule interactions in real time.

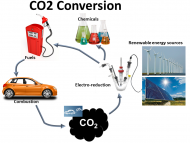
- CO2 Electroconversion into green Fuels:
CO2 emissions resulting from the excessive use of traditional energy sources are the major contributor to global warming. In this respect, electrochemical CO2 conversion into valuable chemicals and fuels with enhanced energy density is a promising solution to mitigate of these environmental changes as well as reduce our dependence on fossil fuels. Unfortunately, the CO2 electroreduction is significantly limited by its very low kinetics (i.e., high overpotential) as well as low energy efficiency, catalyst stability, and product selectivity along with low faradaic efficiency. Thus, our research group is focusing on the design of efficient non-precious nanostructured electrocatalysts with unique morphologies to overcome the inherent CO2 inactivity. Additionally, various advanced in-situ and operando spectroscopy techniques are used to correlate the activity, selectivity and stability to the catalyst structure and reveal detailed chemical information about the catalyst–molecule interactions in real time.
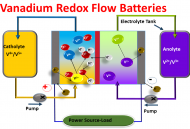
- Vanadium Redox Flow Batteries:
Redox-flow batteries are good choice for storing excess renewable energy (wind and solar energy) in a flexible way are. These systems offer the advantage that energy and power density can be scaled independently of each other at low maintenance cost and with only negligible self-discharge. In the all-vanadium redox flow technology (VRFB) the four oxidation states of vanadium are utilized with the additional advantage that cross-over of cations through the membrane does not lead to severe drawbacks. However, unfortunately, their commercial electrodes (graphite felts) poor electrochemical activity and stability, limiting the power density of the battery, are limited their world-wide commercialization. Consequently, we are working on improve these commercial felts performance (stability and activity) via modifying their fibers with metal/metal oxides nanostructures and introducing a novel carbon nanostructured felts by electrospinning of different polymer solutions.
-Electrocatalysis: Water Splitting, Oxygen evolution, and reduction reactions






